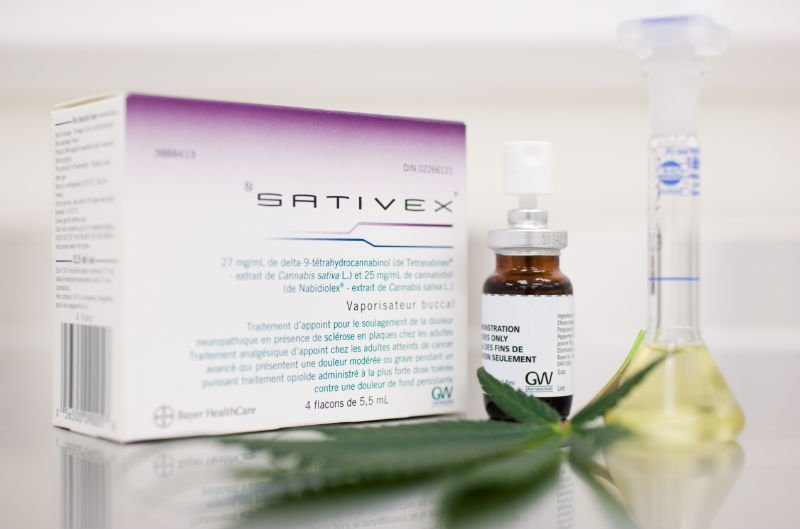
New regulations to approve the use of the cannabis based medicine ‘Sativex’ in the GHA has been published. The new regulations amend the 2005 Drugs Misuse Regulations to allow for the drug to be made available.
This comes a week after a GBC Viewpoint programme in which the use of cannabis in healthcare was discussed.
In the UK the Medicines and Healthcare products Regulatory Agency issued a marketing authorisation for Sativex, for symptom improvement in patients with spasticity due to multiple sclerosis. It followed successful trials to confirm its effitiveness.
Healthcare access to this medicine will now be available under the new regulatory framework.
For more information about this article visit gbc.gi